Hi!
Subscribe to Our Newsletter
Trust us we hate spamming,
you will get delightful emails
September, 2022
Introduction:
The pharmaceutical industry is highly regulated, and this means a lot of communication with regulatory agencies. Regulatory affairs communication deals with the submission of documents and data to regulatory agencies such as the U.S. Food and Drug Administration (FDA) or European Medicines Agency (EMA). You'll often see it abbreviated in emails, reports, memos or other communications as RA-COM or simply "regulatory." However you pronounce it, RA-COM has traditionally been done manually: emails percolate through layers of managers until they reach an RA specialist who then creates a document that is sent back up through the layers before being sent off again via email with an updated timestamp! This kind of roundabout process can take days or even weeks depending on how many people are involved and how urgent the matter is—but now there's another option open to us thanks to automation technology.
Product approvals are obtained through regular regulatory correspondence between big pharma and regulators
Product approvals are obtained through regular regulatory correspondence between big pharma and regulators. There are many documents exchanged between both parties before a product is approved. Pharma companies spend enormous amounts of money in tracking these documents, performing business process activities such as filing, classifying and storing them in a compliant manner. The manual effort performed by regulatory affairs associates can be replaced by cognitive tools and automation if companies implement the right technology. By enabling end-to-end automation in regulatory affairs communication, big pharma companies can
There are many documents exchanged between both parties before a product is approved
Before a pharmaceutical product is approved, there are many documents exchanged between both parties. These are vital for the regulatory affairs associates to understand their role in this process and to ensure that they complete all necessary tasks in time. The documents include:
Tracking documents is a time consuming task for pharmaceutical companies. The process of tracking these documents, performing business process activities such as filing, classifying and storing them in a compliant manner is costly.
Communication automation is the need of the hour in pharmaceutical regulatory affairs
Pharmaceutical regulatory affairs communication is a complex process with the potential to become inefficient. The process involves a number of stakeholders from across the organization, each with their own unique requirements and processes.
These stakeholders include:
With so many stakeholders involved in pharmaceutical product approvals, it’s important that these individuals can share information at all levels in order to keep everyone on the same page.
However, this can be challenging due to the fact that every stakeholder operates differently when handling product communication. For example, some teams may use email for daily updates while others prefer phone calls or text messages because some members may not be able to access emails during certain hours.
Additionally, some teams might use paper-based documentation while others rely on digital systems like SharePoint or Confluence. As such, there’s no central repository that contains all communications related to one product which makes it difficult for all parties involved to stay informed about upcoming deadlines or changes in strategy which could have an impact on production timelines or logistics planning. This also leads to duplication of work since every stakeholder within your organization will need it.
With a huge number of products and processes, it is impossible to keep track of all the regulatory documents at one go.
Pharmaceutical companies have to comply with multiple regulations and standards, which further add to the complexity of regulatory documentation management. As discussed earlier, with time and budget constraints, it is not possible for every stakeholder involved in the process to keep an eye on everything. Therefore, it’s mandatory for organizations to automate their regulatory communications processes so that they can gain a competitive advantage through timely and accurate regulatory submissions.
The manual effort performed by regulatory affairs associates can be replaced by cognitive tools and automation if companies implement the right technology.
ACE, or Algonox Cognitive Engine, is an end-to-end solution that provides a single view of all company data across the enterprise and makes it easy to update information in multiple systems at once. The platform helps automate nonvalue added tasks like documentation gathering, reporting and approvals.
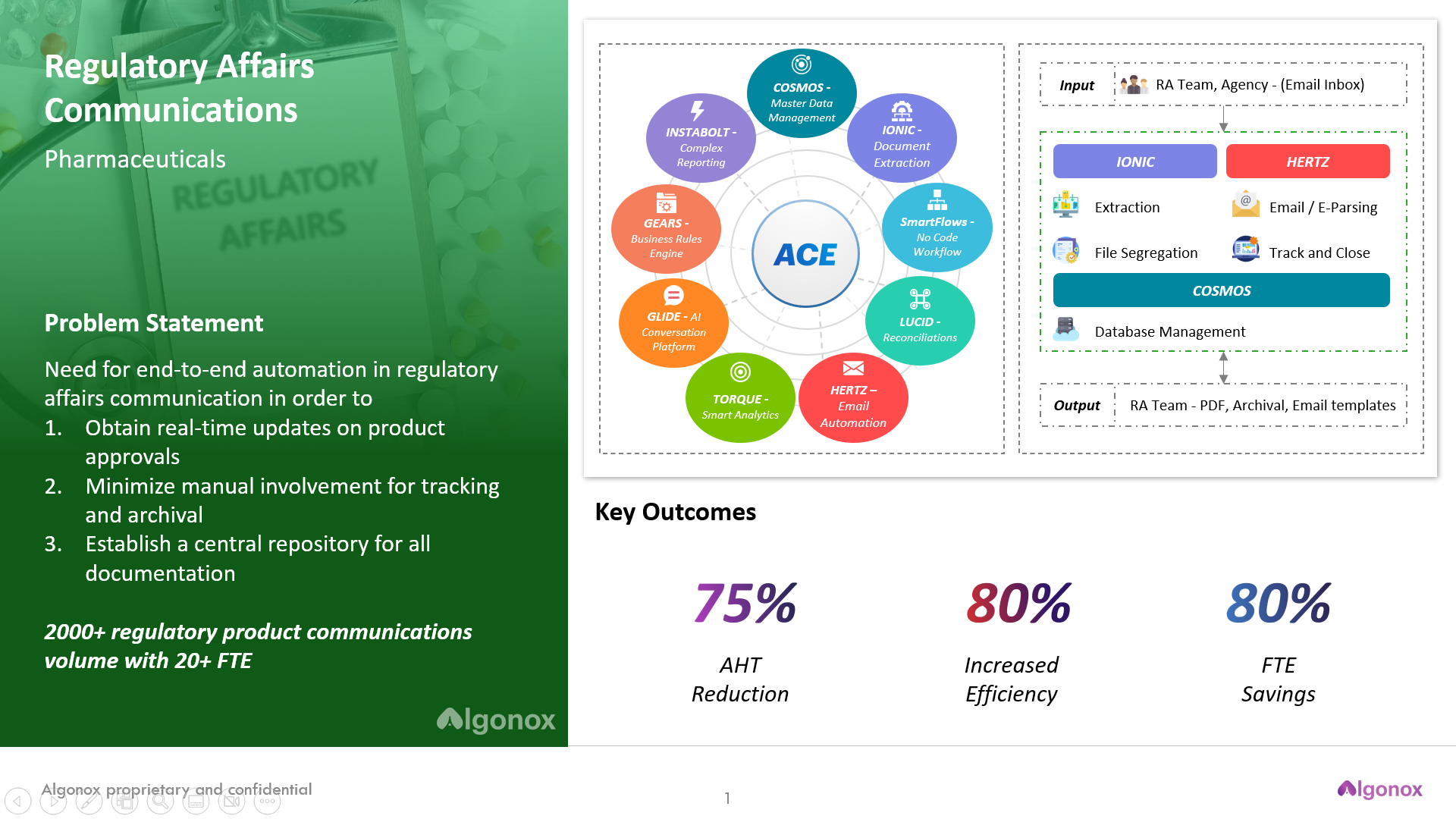
Conclusion:
It is important to note that automation can be used by both parties in the global regulatory affairs communication. It helps pharma companies to minimize manual efforts and reduce operational costs. On the other hand, it also helps regulators by streamlining their operations and centralizing all documents in a central repository where they can easily find them when needed. Regulators are continuously adopting new technologies to streamline their operations while ensuring compliance with all industry standards and regulations. This will help big pharma companies get approvals faster and keep pace with the fast growing healthcare market.
